BiMobile Dual Mobility System

Reliable - Safe - Solution
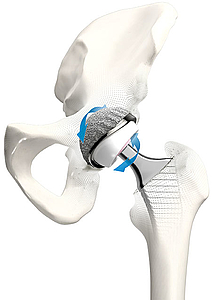
The concept of double mobility was developed by Prof. Gilles Bousquet in 1975 with the aim of treating habitual hip luxation.13 The system consists of a metal casing with a highly polished internal surface and a movable polyethylene inlay, in which a press-fitted prosthesis head moves. This provides a greater range of motion with less abrasion14, 15, 16 and reduced risk of luxation.15, 17, 18 It was on the basis of this principle that the BiMobile Acetabular Cup System came about.
The development of the bimobile acetabular cup system drew on many years of experience with successful implant systems and fixation concepts plus state-of-the-art material and coating technologies. The result is the versatile BiMobile Acetabular Cup System.
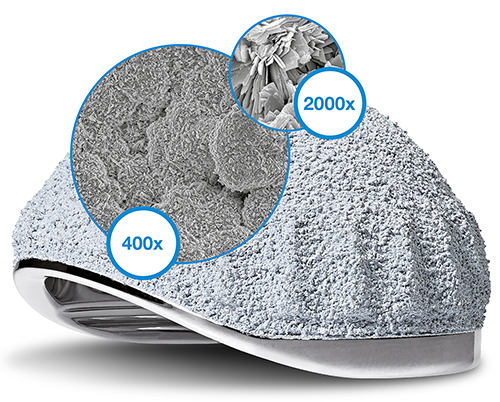
The cementless BiMobile Acetabular Cup is available with a TiCaP double coating. The TiCaP double coating combines the properties of a highly porous layer of pure titanium for primary fixation and calcium phosphate coating, which together provide optimal primary and secondary implant stability.4, 5 A special macrostructure on the cup equator increases primary stability.9
Highly wear-resistant acetabular cup1, 2, 6
The BiMobile Dual Mobility System is available in two versions, either cemented or cementless. The metal casings in both versions are fabricated from biocompatible, sturdy EndoDur CoCrMo material.1, 2 The inner surface is highly polished in order to minimize wear.
Use of known anchoring techniques
The cemented BiMobile Acetabular Cup has a finely matt-finished SatinLink surface, which is also a feature of the SP II stems. Latitudinal and longitudinal groove-like structures reinforce the fixation and allow air to escape when the implant is pressed into the cement bed.

Self-centering inlay11

The inlays are available in UHMWPE and E-Dur (X-LINKed Vit-E PE) and can be combined with Link prosthesis heads made of CoCrMo or ceramic with a 22 or 28 mm diameter.
Features and advantages
Highly abrasion-resistant, biocompatible EndoDur CoCrMo material1, 2, 6
Safe implantation thanks to a fixed implant-instrument connection and unobstructed view of the acetabular cup rim11, 12
Cementless and cemented fixation
28 mm prosthesis heads starting at a cup size of 48 mm for a wide range of motion
Broad range of sizes (42–70 mm)
Clinically proven, very rough TiCaP double coating2, 3
Self-centering inlays for a uniform load distribution and increased protection against luxation7
Inlays available in Vit-E PE and UHMWPE
Anatomical medioventral recess for a wide range of motion and for protection of the femoral nerve and iliopsoas
Size-adapted clearance between liner and metal casing for constant articulation11
Intraoperative flexibility11, 12
- Internal document W. LINK (DOC-08614)
- Internal document W. LINK (DOC-08725)
- Ullmark G et al.: "Analysis of bone formation on porous and calcium phosphate-coated acetabular cups: a randomised clinical [18F] fluoride PET study." Hip International 22.2 (2012).
- Cunningham B W et al.: “General Principles of Total Disc Replacement Arthroplasty”, Spine, Vol. 28, No. 20 Suppl., 2003
- Bobyn, J. D., et al. „The optimum pore size for the fixation of porous-surfaced metal implants by the ingrowth of bone.“ Clinical orthopaedics and related research 150 (1980): 263-270.
- Long, M., & Rack, H. (1998). Titanium alloys in total joint replacement—a materials science perspective. Biomaterials, 19(18), 1621-1639.
- Fabry C, Kaehler M, Hermann S, Woernle C, Bader R. 2014. Dynamic behavior of tripolar hip endoprostheses under physiological conditions and their effect on stability. Medical Engineering & Physics 36:65- 71.
- Internal document W. LINK (DOC-08553)
- Internal document W. LINK (DOC-08695)
- Loving L, Herrera L, Banerjee S, Heffernan C, Nevelos J, Markel DC, Mont MA. 2015. Dual mobility bearings withstand loading from steeper cup-inclinations without substantial wear. J Orthop Res. 33(3):398-404.
- Internal document W. LINK (DOC-08847)
- Internal document W. LINK (DOC-07974)
- Noyer, D., Canton, J. H. (2016). Once upon a time… Dual mobility: hi story. International Orthopaedics Vol. 41 - No. 3 (March 2017): 611-618
- Charnley, John. „The long-term results of low-friction arthroplasty of the hip performed as a primary intervention.“ Bone & Joint Journal 54.1 (1972): 61-76.
- Philippot, R., Camilleri, J. P., Boyer, B., et al. (2009). The use of a dual-articulation acetabular cup system to prevent dislocation after primary total hip arthroplasty : analysis of 384 cases at a mean follow-up of 15 years . SICOT 33: 927-932.
- Wroblewski, B., Siney, P., Flemin, P. (2009). The principle of low frictional torque in the Charnley total hip replacement. JBJS (Br) Vol. 91-B(7): 855-858.
- Stroh, D. Alex, et al. "Dual-mobility bearings: a review of the literature." Expert review of medical devices 9.1 (2012): 23-31.
- Nevelos, J., Bhimji, S., Macintyre, J., et al. (2010). Acetabular Bearing Design Has a Greater Influence on Jump Distance than Head Size. 56th Annual ORS Meeting: Poster #2028.